Clinical Trials
Clinical trials ensure that safe, effective medicines make it to the people who need them.
What is a clinical trial?
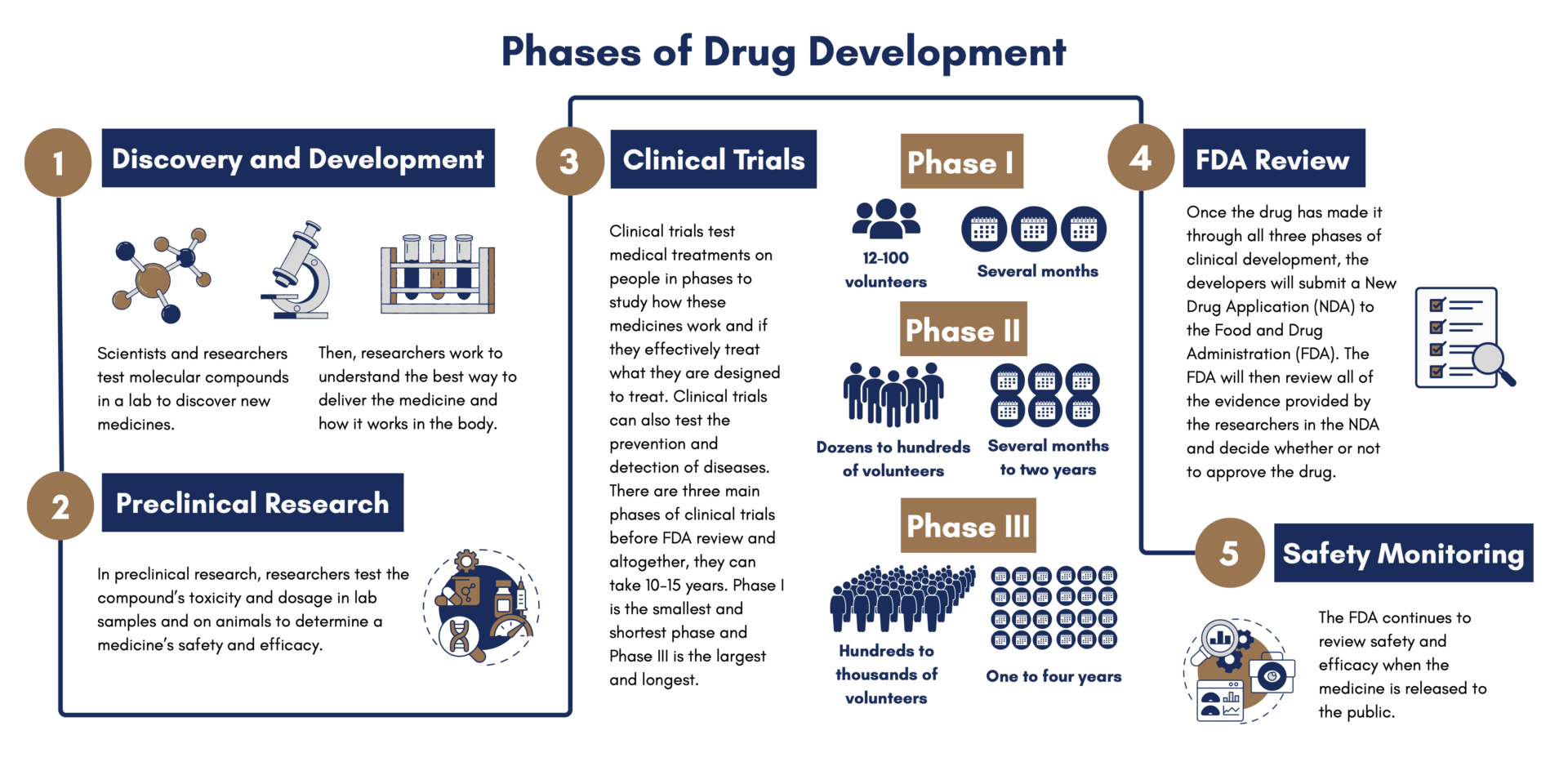
Clinical trials are just one of the many steps involved in getting a medicine from the lab and into patients’ hands. First, in the discovery and development stage, scientists and researchers test molecular compounds, and then, in the preclinical stage, researchers test the compound’s toxicity and dosage on animals. Clinical trials test medical treatments on people in phases to study how these medicines work and if they effectively treat what they are designed to treat. Clinical trials can also test the prevention and detection of diseases.
Only about 8% of investigational new drugs that begin clinical testing make it through all stages and reach regulatory approval. In some cases, the process can take about 10-15 years, though for rare diseases like cystic fibrosis, drugs can move faster.
For drug makers, it is a time and resource intensive process. For the people who enroll in clinical trials, often referred to as participants, it is often a deeply personal decision to do so. For researchers and clinicians who rely on clinical trials to advance the science about a given medical condition, clinical trials represent the final part of the drug development process that brings new medicines from the test tube to the patient.
What are the phases of clinical trials?
Phase 1 – This phase includes several dozen to one hundred volunteers and takes several months to complete. About 70% of drugs move through this phase. The goal of this phase is to learn about the drug’s safety and side effects. Sometimes, these participants are “normal healthy volunteers” rather than people who live with a given medical condition. Other times, patients themselves can be included in phase I trials.
Phase 2 – This phase includes many dozen to several hundred people and can last from several months to two years. About one third of drugs move through this phase. The goal of this phase is to see how effective the treatment is and to continue to monitor its safety.
Phase 3 – This phase can include up to several hundred to thousands of volunteers and can last one to four years. About one quarter of drugs make it through this phase. The goal of this phase is to confirm how effective the drug is and to compare it with other treatments.
If Phase 3 is successful, drugs can be approved by the FDA.
Phase 4 – This phase continually monitors safety and efficacy after the FDA approves the drug and it becomes publicly available.
Are clinical trials safe?
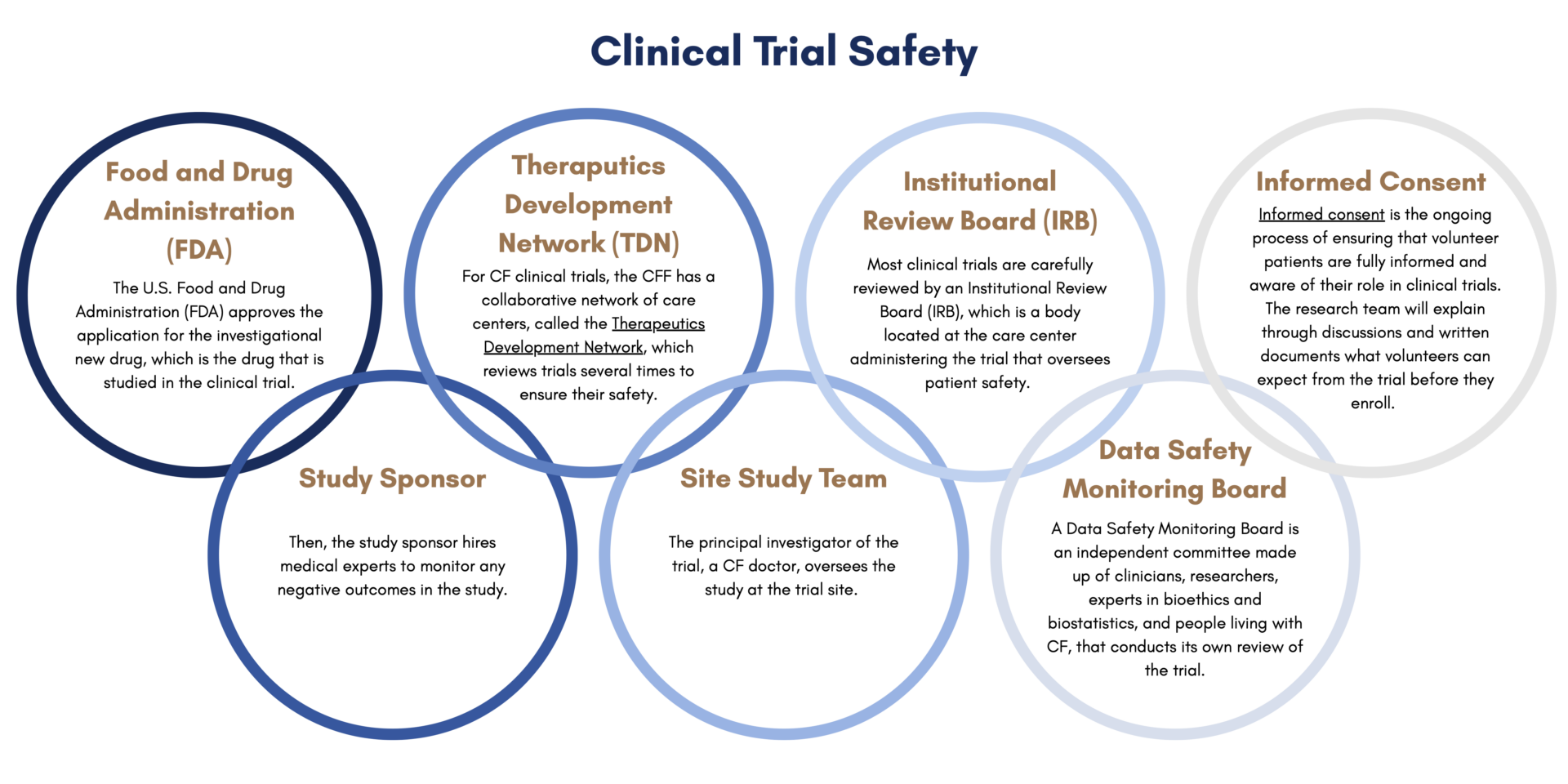
Before patients start a clinical trial, they go through a process called informed consent. Informed consent is the ongoing process of ensuring that volunteer patients are fully informed and aware of their role in clinical trials. The research team will explain through discussions and written documents what volunteers can expect from the trial before they enroll. This includes what the purpose of the trial is, how long it is projected to last, what is required to participate, and the potential risks and benefits.
There are many layers of safety built into the clinical trial process. First, the U.S. Food and Drug Administration (FDA) approves the application for the investigational new drug, which is the drug that is studied in the clinical trial. Then, the study sponsor hires medical experts to monitor any negative outcomes in the study. For CF clinical trials, the Cystic Fibrosis Foundation has a collaborative network of care centers, called the Therapeutics Development Network, which reviews trials several times to ensure their safety. Clinical trials also have a Data Safety Monitoring Board, which is an independent committee made up of clinicians, researchers, experts in bioethics and biostatistics, and people living with CF, that conducts its own review of the trial. Most clinical trials are carefully reviewed by an Institutional Review Board (IRB), which is a body located at the care center administering the trial that oversees patient safety. The principal investigator of the trial, a CF doctor, oversees the study at the trial site. CFF has a graphic that explains how these layers of safety work together.
What can I expect from participating in a clinical trial?
Every clinical trial is different depending on the treatment that is being tested. Before enrolling in a clinical trial, patients will have the opportunity to review the trial’s protocol, which is a document that explains what will go on during the trial and why. The protocol will list all of the relevant information regarding participation, including how long the trial will last and what volunteers are expected to do.
Who are the team members in a clinical trial?
More FAQS
The eligibility criteria for a clinical trial will be clearly listed on the trial protocol, which is a document that explains what will go on during the trial and why. Eligibility criteria can include age, type of CFTR mutation, health status, treatment status, and what other conditions the patient has.
Sponsors, such as pharmaceutical companies, academic institutions, government agencies, and nonprofits, provide funding for clinical trials.
Clinical trials are completely voluntary and volunteers can leave at any time.
How do I find a clinical trial?
About pRxEngage
pRxEngage is a platform that helps you discover research studies that could be right for you. You can search by condition or location, browse easy-to-read trial profiles, and answer short assessments that highlight studies that might fit both your medical situation and your lifestyle.
If you find a study that interests you, you can save it, share it with your doctor, or request more information. At that point, if you choose to proceed, the research site running the study takes over. pRxEngage itself does not recruit you into trials. Nothing happens without your permission, and you are always in control of the process.
Your privacy is central. pRxEngage only asks for the information needed to help match you to relevant studies. That information is stored securely and is never sold.
Your data is only shared with a research site if you choose to apply for a trial and give permission for your details to be passed on. At any point, you can ask for your information to be deleted.
For full details, you can read pRxEngage’s Privacy Policy. In short, your information is yours, and you control what happens to it.
All trials listed on pRxEngage have already been reviewed and approved by regulators and ethics committees, which means they have been assessed for safety and scientific value. It is important to be clear that all clinical trials carry risks as well as potential benefits.
pRxEngage provides plain-language summaries of each study, including brief information about possible risks and benefits. We also provide a guide with the kinds of questions you should ask the research team before deciding. The full patient information sheet for each trial lists the risks and benefits in detail, and we always encourage you to read it carefully.
Compatibility is about more than eligibility. It is also about whether you feel ready and able to take on the commitments of the study. That is why pRxEngage looks at both medical fit and mental fitness, meaning your ability to cope with the demands of research. The final decision is always made by the research site, and we strongly encourage you to discuss any study you are considering with your current care team before making a decision.
pRxEngage does not recruit patients into trials. We support awareness and engagement by helping you understand your options and access the right information. If you choose to move forward, the research site running the study will handle all screening and next steps. With your consent, they may contact your physician to confirm your medical history.
On your side, pRxEngage makes it easy to share study details with your care team. You can download or print the study profile and bring it to your next appointment. That way, your doctor or specialist is fully aware and can advise you.
If anything is unclear, pRxEngage is always here to support you. No question is too small.
No. The Foundation is not being paid to recommend or push patients into any particular study. The purpose of hosting pRxEngage is to give patients and families a trusted, simple way to learn about all ongoing clinical trials.
Some of the trials listed have a commercial relationship with pRxEngage, but many do not. All trials are treated equally on the platform. For transparency, if a patient finds a study through pRxEngage and goes on to screening for a trial where there is a commercial agreement in place with the sponsor, a $250 payment is passed from pRxEngage to the Foundation. This is not a payment for referral. It is a way of sharing value with community groups who help make patients aware of research opportunities.
Neither the Foundation nor pRxEngage recruits patients or makes money from simply sending patients through. The decision to join a study is always yours. The reason the widget is hosted here is simple: both pRxEngage and the Foundation share a deep commitment to making research easier to understand, more accessible, and more supportive of patients.
